WEST COLUMBIA, S.C. — Local pharmaceutical corporation Nephron was recently flagged by the Canadian Health Agency for quality control and sterilization issues of their products.
Canadian Health recently released a report calling West Columbia drug manufacturer Nephron Pharmaceuticals inadequate. This comes after inspectors from the Canadian Health Agency visited the facility in October.
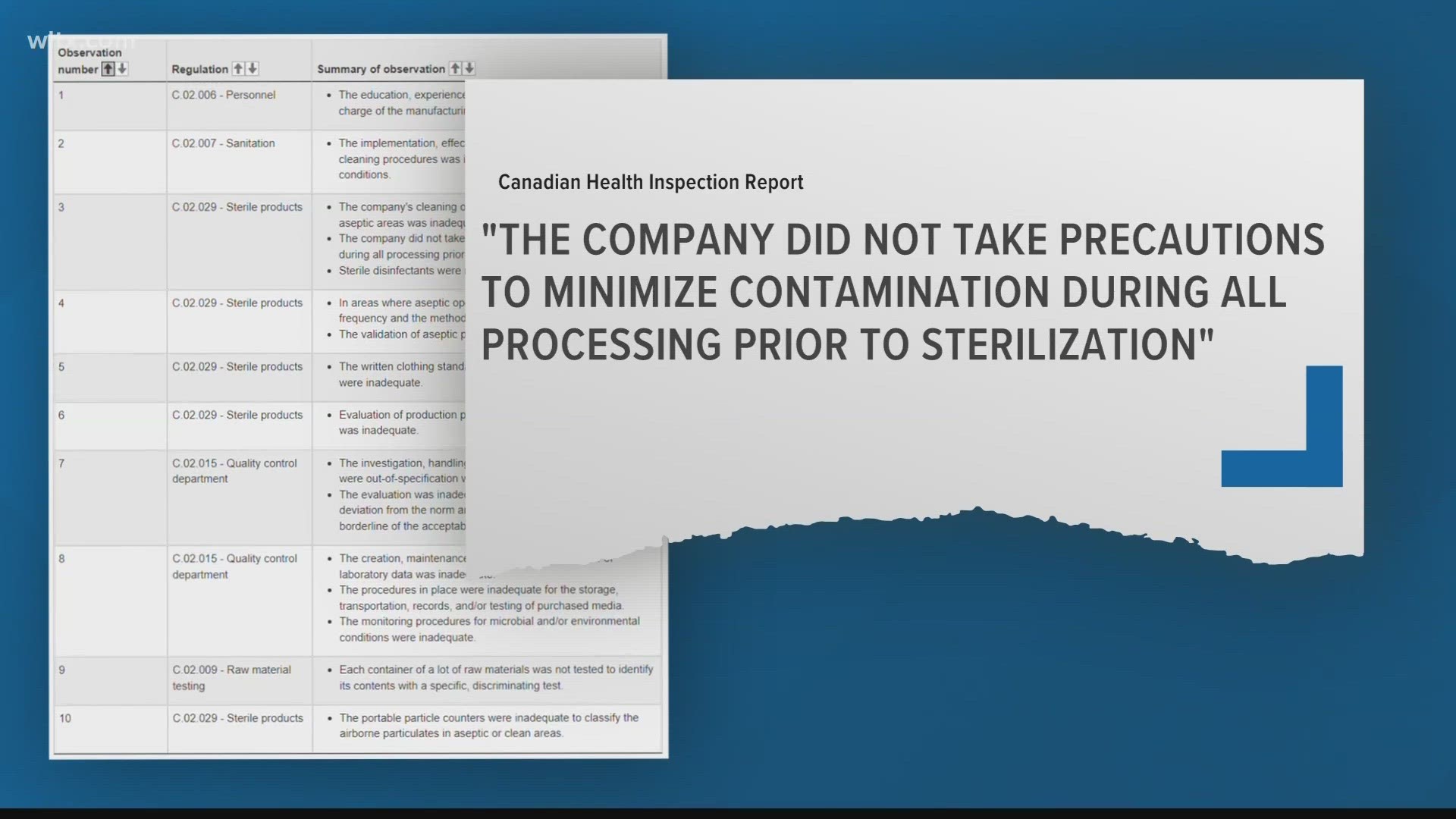
In the report, Canada Health, gives the facility an overall “non compliant” rating and highlighted several issues with the company’s process of making health products.
The report examined multiple areas such as, sanitation, personnel and the sterilization of products. It states “the company did not take precautions to minimize contamination during all processing prior to sterilization."
It also noted that the “the education, experience and/or oversight of the individual in charge of the manufacturing department was inadequate."
In response to the report, Nephron said in a statement to News 19:
“Since Canada Health visited, the FDA has been back to follow up with Nephron. We worked closely with them, as we always do, and we await their conclusions, given that the results of the FDA will serve as the current company inspection status.”
The Canadian Health Agency inspected Nephron’s facility as part of its regulation of pharmaceuticals available in Canada. This is to make sure the drugs and products made in America are compatible with Canadian law.
This is not the first time the company has faced issues in their inspections. In 2023, the FDA noted problems with Nephron’s equipment and maintenance, improper storage of ingredients and employees lacking the proper training.
In another statement to News 19, the company said, in part, “Nephron continues to work collaboratively with FDA or any other agency to resolve any questions or concerns regulators have about company processes."